This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
LDCT screening pilots in Poland
Poland has made important contributions to the lung cancer screening agenda in Europe since it first initiated implementation research into LDCT screening in 2008.1 To date, pilots in Szczecin, Gdańsk, Poznań and Warsaw have screened almost 50,000 people in total: approximately 1% were confirmed to have lung cancer, and between 64% and 70% were diagnosed at stage I.2 This is important, as there are more options for treatment of early-stage lung cancer (e.g. curative surgery), which improves survival.
Each pilot was short term, limited to 1–3 screening examinations per participant and had a distinct source of funding. Nonetheless, they have provided important lessons around the implementation of LDCT screening in Poland and culminated in the publication of a Polish consensus statement for the implementation of a national programme in 2018.2
A short summary of LDCT screening pilots in Poland
- Silesia: screened 602 people from Katowice and Zabrze; funded by local government.3-5
- Poznań: screened 17,222 people across the Wielkopolskie voivodeship between 2009 and 2015. The programme was funded by regional health authorities.6
- Szczecin: targeted people aged 55–65 who were invited through a personalised invitation letter sent by the Szczecin City Mayor.7
- Warsaw: targeted the districts with the lowest surgical resection rates; financed by the Ministry of Health.8
- Gdańsk: two pilot programmes financed by national scientific funds screened 15,200 individuals in total. The MOLTEST BIS was a regional pilot funded by grants from the National Centre for Research and Development.8 9 It followed on from an earlier pilot, the Pomeranian Pilot Program for Lung Cancer Screening, which was financed by the European Economic Area Financial Mechanism and the Marshall of the Pomeranian Voivodeship.
The national programme in Poland
Poland is the second country in Europe to implement a nationwide LDCT screening programme for lung cancer,10 11 a protocol for which was published in 2019. The National Program of Early Lung Cancer Detection (Ogólnopolski Program Wczesnego Wykrywania Raka Płuca, WWRP) officially began in 2020.
As a centrally administered programme co-financed by the Ministry of Health and the European Social Fund,12 it is currently being rolled out in a phased approach. Over the course of three years, it seeks to enrol 16,000 participants aged 50–74 across six macroregions in Poland.
Six research centres have been selected to lead the implementation. The aim is that, as the WWRP expands, these become ‘centres of excellence’ that can provide guidance to other sites on lessons learnt from implementation:
| Area of Poland | Leading centre |
| Central | Institute of Tuberculosis and Lung Diseases in Warsaw |
| East | Białystok Oncology Center |
| North | Medical University of Gdańsk |
| West | Poznań Medical University |
| Silesia (South) | Medical University of Silesia |
| South-east | Świętokrzyskie Oncology Centre |
To support delivery of the programme, a cloud-based platform designed in collaboration with the International Early Lung Cancer Action Project (I-ELCAP) was rolled out to all participating sites. The platform centralises epidemiological and clinical data collection, and houses resources to support screening staff, including guidelines for radiologist decision-making and a range of interactive tools (e.g. risk-prediction models to guide eligibility for screening).
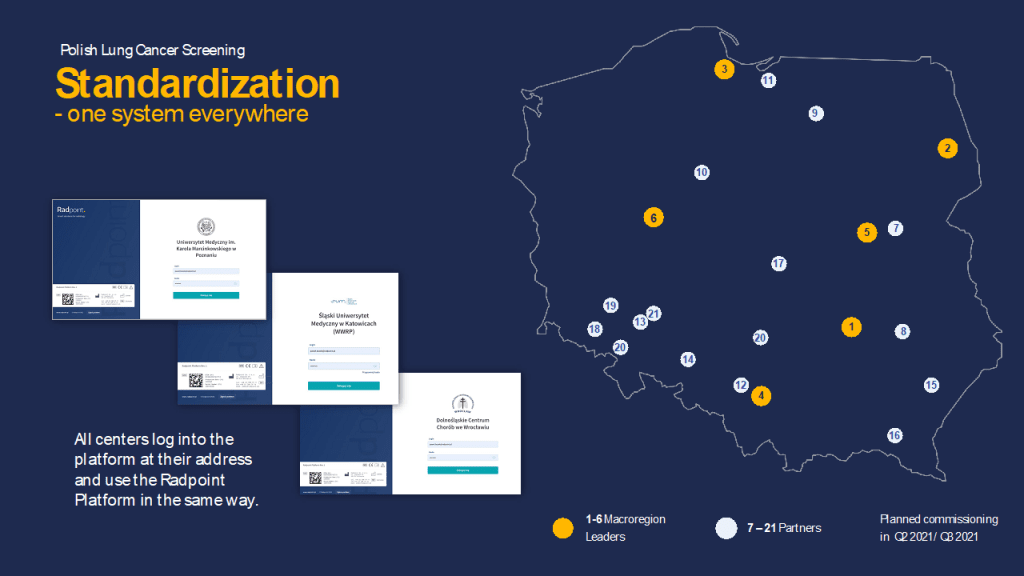
A map of the partners and subcontractors currently participating in the Polish national lung cancer screening programme13
Next steps for screening in Poland
Smoking remains an important challenge in Poland.14 The Ministry of Health runs a prevention programme for tobacco-related diseases, which involves counselling via smoking cessation clinics.12 However, embedding smoking cessation within the screening pathway is an area of ongoing development.13 It has been rolled out successfully in some WWRP sites following a tailored approach, although not all sites currently have access to a smoking cessation clinic.
Challenges have also been caused by the COVID-19 pandemic, which resulted in a temporary pause in recruitment in 2020. Interest in participating in the programme also gradually slowed once recruitment resumed. A mass media campaign and strategies to engage healthcare professionals were therefore evaluated to understand which was best to sustain interest. As a result, almost 14,000 people have received LDCT screening via the WWRP to date.
Additions to the cloud-based platform are planned, such as a teaching module for radiologists for quality assurance. Partner institutions also provide computer-aided detection (CADe) software that utilises artificial intelligence (AI) to support the evaluation of scans.15
The first term of the WWRP is due to end in 2023. To secure funding for a new term, the programme leads aim to build better awareness of the benefits of LDCT screening and develop resources designed to ensure that delivery aligns with workforce capacity within the programme. Implementation studies are also ongoing to assess the role of biomarkers in supporting the selection of a population at high risk of lung cancer for screening.16 17
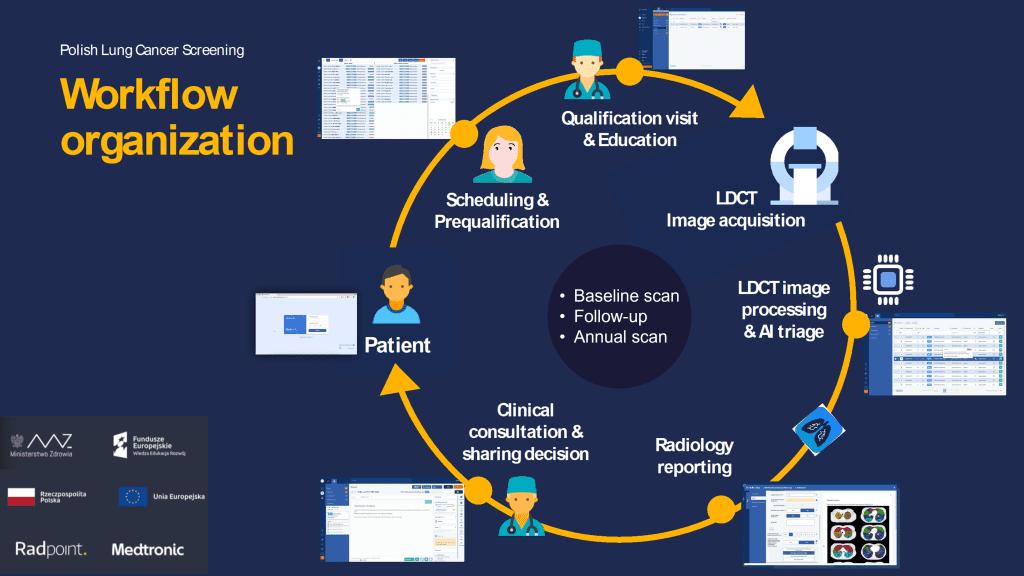
The participant pathway in the Polish national lung cancer screening programme
The Lung Cancer Policy Network is publishing brief case studies of countries that have implemented LDCT screening; you can read other examples here.
We will also continue to build the extensive implementation research in Poland into the second edition of the map.
Recent news
Case study

Implementing a national screening programme for lung cancer in South Korea
With insights from Network member Yeon Wook Kim, we look at the key steps taken to implement the national screening programme for lung cancer in South Korea.
News

Join European lung cancer experts in Paris to explore how screening implementation can be optimised
On 27 March, the Lung Cancer Policy Network will host a panel discussion and networking event to coincide with the European Lung Cancer Congress (ELCC) 2025.
Case study

Lung cancer screening in the Middle East and Africa: using the Network framework to facilitate implementation
The Lung Ambition Alliance Middle East and Africa (LAA MEA) Chapter has used the Network’s framework to develop recommendations across priority areas for lung cancer screening in the region.
References
Adamek M, Biernat W, Chorostowska-Wynimko J, et al. 2020. Lung cancer in Poland. Journal of Thoracic Oncology 15(8): 1271-76
Rzyman W, Didkowska J, Dziedzic R, et al. 2018. Consensus statement on a screening programme for the detection of early lung cancer in Poland. Advances in Respiratory Medicine 86(1): 53-74
Szablowska-Siwik S, Wachula E, Czyzewski D, et al. 2017. Lung cancer screening with LDCT; Results of a small cohort continual monitoring (Pilot Silesian Study). Journal of Thoracic Oncology 12(11): S2389-S90
Wachuła E, Szabłowska-Siwik S, Czyżewski D, et al. 2020. Emphysema affects the number and characteristics of solitary pulmonary nodules identified by chest low-dose computed tomography. A study on screenees with high-risk lung cancer recruited in Upper Silesia. Pol Arch Intern Med 130(1): 17-24
Wachuła E, Szabłowska-Siwik S, Czyżewski D, et al. 2020. Retrospective assessment of Lung-RADS® performance in the Silesian Lung Cancer Screening Pilot Study. Oncology in Clinical Practice 16(2): 52-55
Bryl M, Nikisch B, Dyszkiewicz W, et al. 2017. Implementation of LDCT lung cancer screening into practice. Results of regional early detection program. Journal of Thoracic Oncology 12(1): S347-S48
Kołaczyk K, Walecka A, Grodzki T, et al. 2014. The assessment of the role of baseline low-dose CT scan in patients at high risk of lung cancer. Polish Journal of Radiology 79: 210-18
Rzyman W. Lung cancer screening in Poland pilot program is restarted after COVID-19 lockdown. [Updated 20/01/21]. Available from: https://www.iaslc.org/iaslc-news/ilcn/lung-cancer-screening-poland-pilot-program-restarted-after-covid-19-lockdown [Accessed 28/01/21]
Ostrowski M, Marjański T, Dziedzic R, et al. 2019. Ten years of experience in lung cancer screening in Gdańsk, Poland: a comparative study of the evaluation and surgical treatment of 14,200 participants of 2 lung cancer screening programmes. Interact Cardiovasc Thorac Surg: 10.1093/icvts/ivz079
Poon C, Haderi A, Roediger A, et al. 2022. Should we screen for lung cancer? A 10-country analysis identifying key decision-making factors. Health Policy 126(9): 879-88
Wait S, Alvarez-Rosete A, Osama T, et al. 2022. Implementing lung cancer screening in Europe: taking a systems approach. JTO Clinical and Research Reports 3(5): 100329
Ministry of Health. 2019. Ogólnopolski Program Wczesnego Wykrywania Raka Płuca (WWRP) za Pomocą Niskodawkowej Tomografii Komputerowej (NDTK) – połączenie prewencji wtórnej z pierwotną w celu poprawy świadomości dotyczącej raka płuca wśród społeczeństwa i personelu ochrony zdrowia. Warsaw: Ministry of Health
Bidzińska J. 2022. Lung cancer screening in Poland: Organizational program set-up. Lung cancer screening in Europe; 27/09/22; Online
Rzyman W. 2019. The implementation of lung cancer screening – The Poland experience. International Association for the Study of Lung Cancer (IASLC) Webinar; 05/03/19; Online
Majewska M. 2022. Can artificial intelligence replace the radiologist? AI in lung cancer screening and teleradiology [online]. Puls Medycyny. Available from: https://pulsmedycyny.pl/czy-sztuczna-inteligencja-moze-zastapic-radiologa-ai-w-skriningu-raka-pluca-i-teleradiologii-1153405 [Accessed 10/10/22]
Widłak P, Jelonek K, Kurczyk A, et al. 2021. Serum metabolite profiles in participants of lung cancer screening study; comparison of two independent cohorts. Cancers 13(11): 2714
Smolarz M, Kurczyk A, Jelonek K, et al. 2021. The lipid composition of serum-derived small extracellular vesicles in participants of a lung cancer screening study. Cancers 13(14): 3414
